Preclinical assessment of an optimized AAV-FVIII vector in mice and non-human primates for the treatment of hemophilia A: Molecular Therapy - Methods & Clinical Development

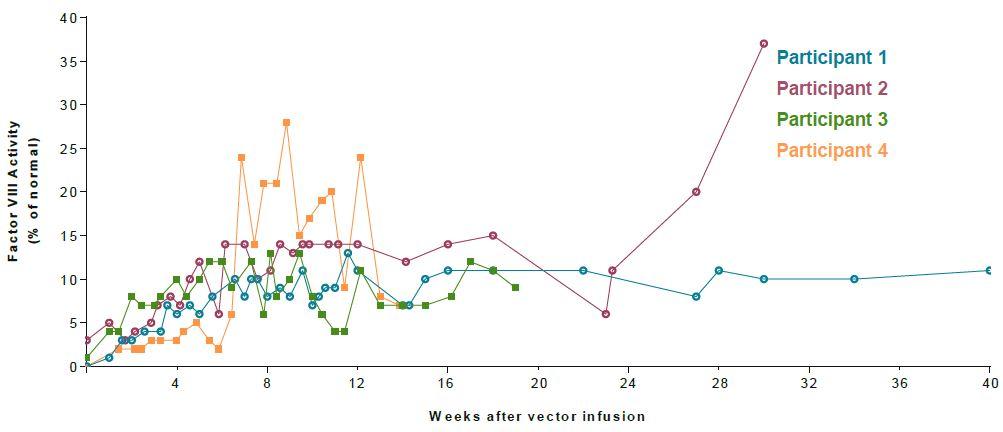
Phase I/II Trial of SPK-8011: Stable and Durable FVIII Expression for > 2 Years With Significant ABR Improvements in Initial Dose Cohorts Following AAV-Mediated FVIII Gene Transfer for Hemophilia A

Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer: Molecular Therapy - Methods & Clinical Development

Spk-8011: Preliminary Results from a Phase 1/2 Dose Escalation Trial of an Investigational AAV-Mediated Gene Therapy for Hemophilia a - ScienceDirect

Phase I/II Trial of SPK-8011: Stable and Durable FVIII Expression for > 2 Years With Significant ABR Improvements in Initial Dose Cohorts Following AAV-Mediated FVIII Gene Transfer for Hemophilia A
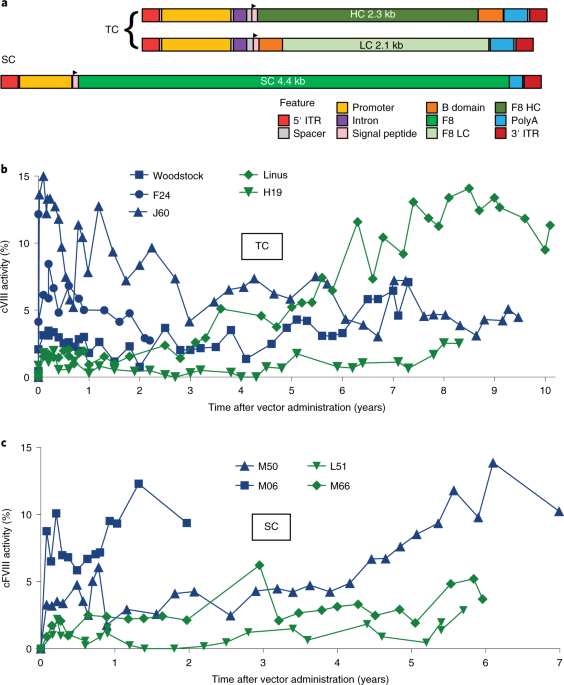
A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells | Nature Biotechnology

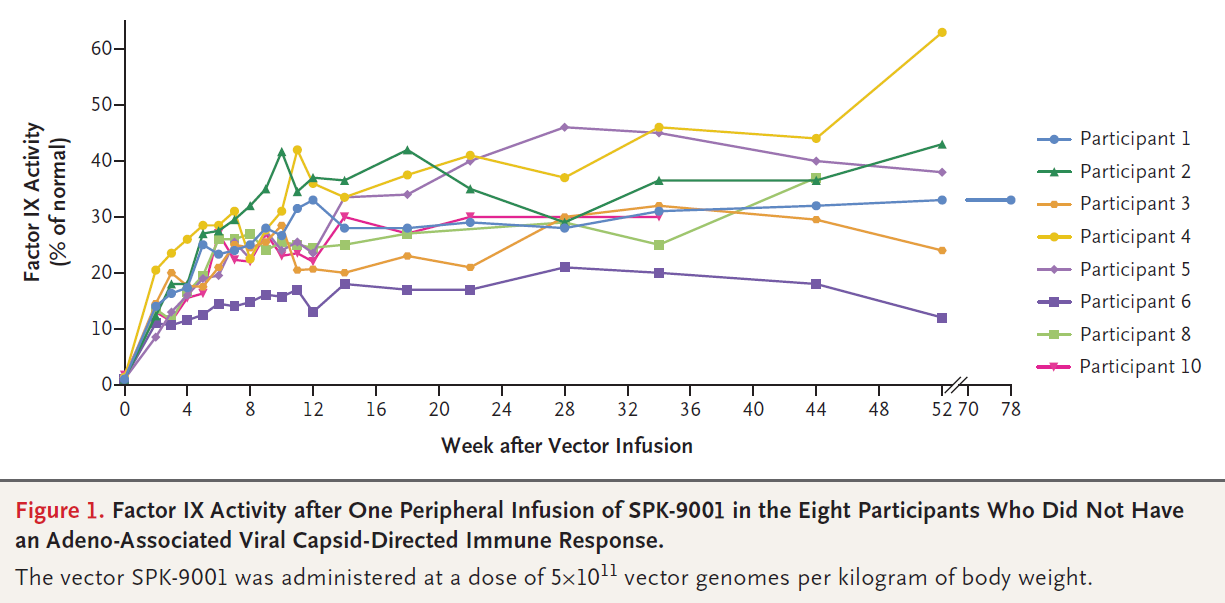
Phase I/II Trial of SPK-8011: Stable and Durable FVIII Expression for > 2 Years With Significant ABR Improvements in Initial Dose Cohorts Following AAV-Mediated FVIII Gene Transfer for Hemophilia A
Hemophilia A Clinical Gene Therapy Trials (September 2020). Listed are... | Download Scientific Diagram