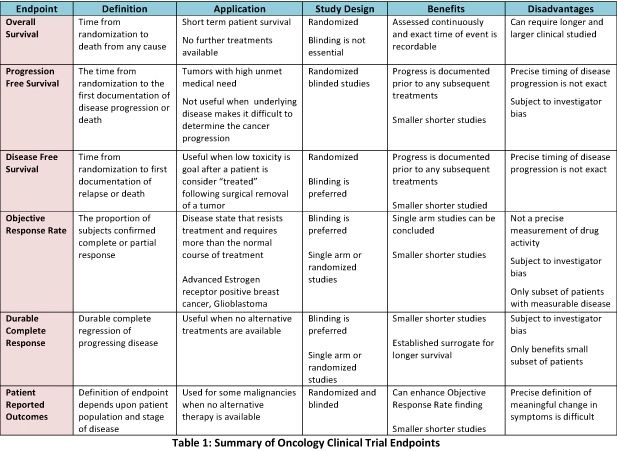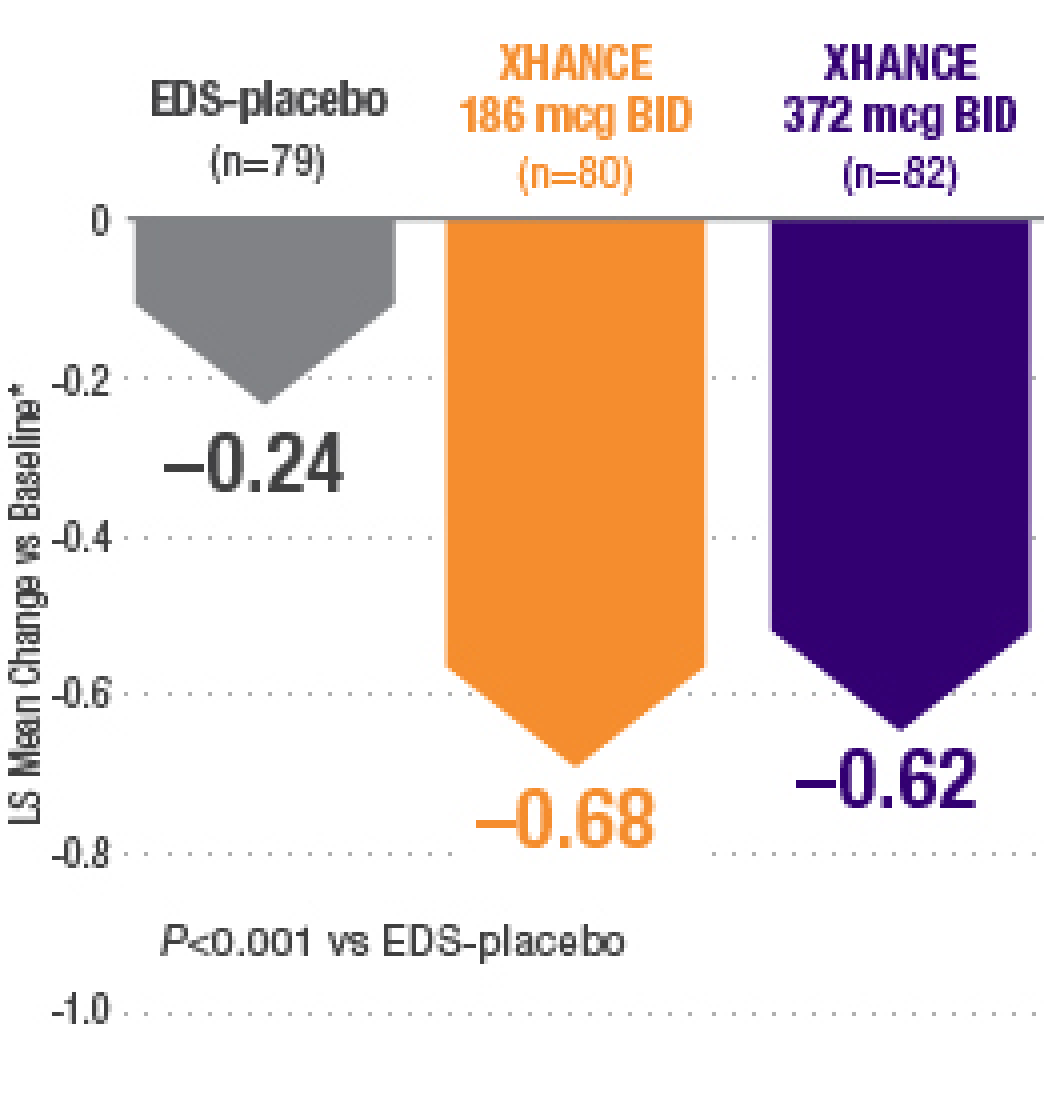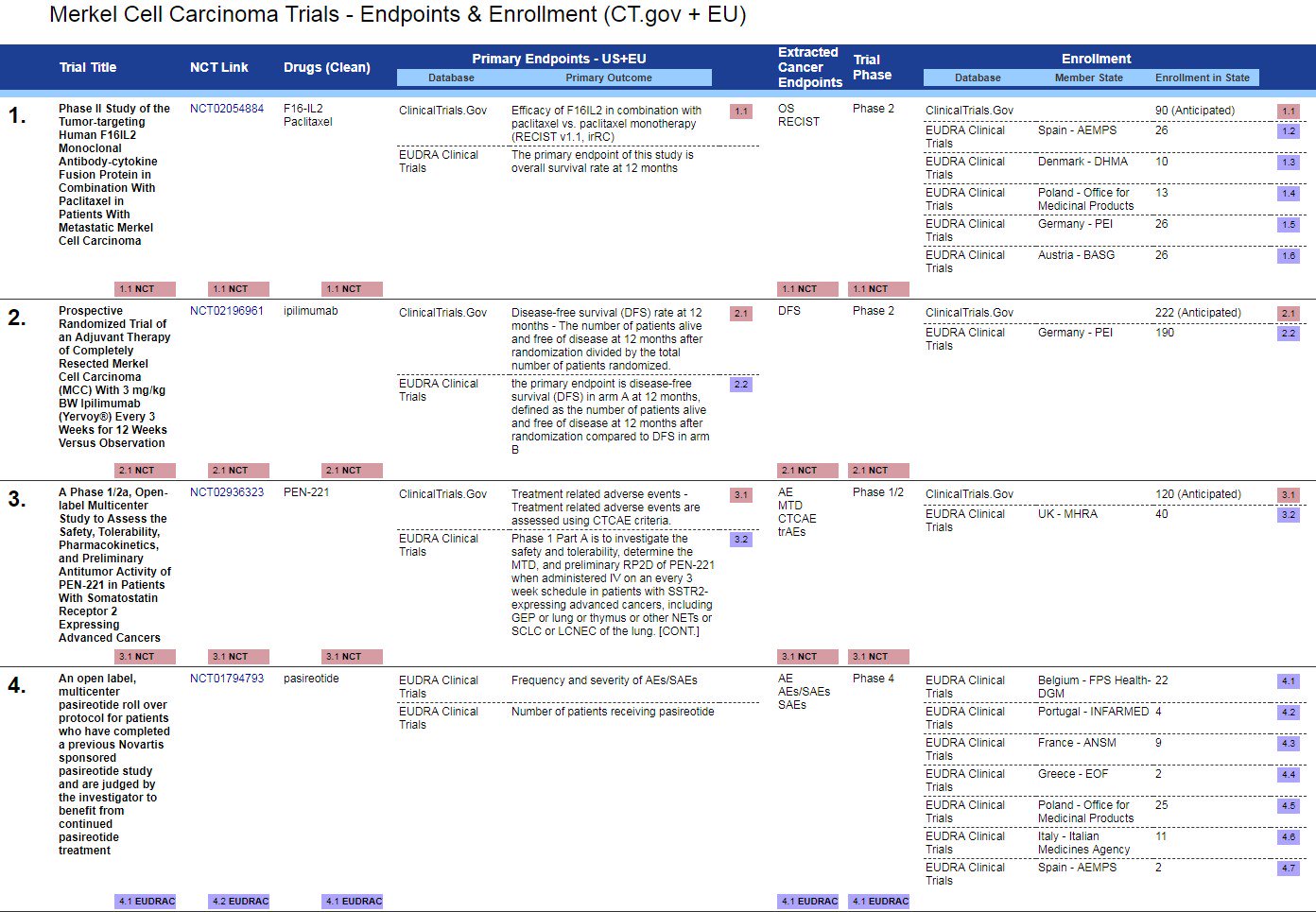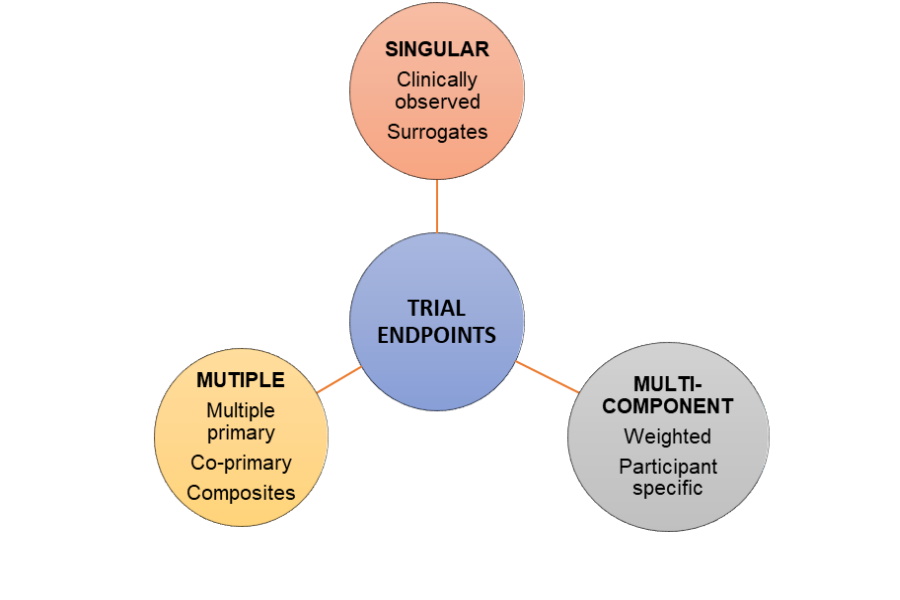
The Endpoint Selection: a Complex Process in the Clinical Trials Design Page CRA School | The International Clinical Research Academy Page | CRA School | The International Clinical Research Academy
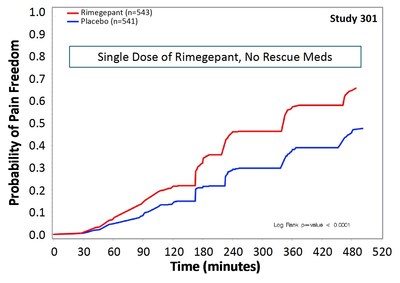
Biohaven Announces Successful Achievement of Both Co-Primary Regulatory Endpoints in Two Pivotal Phase 3 Trials of Rimegepant an Oral CGRP Receptor Antagonist for the Acute Treatment of Migraine | Biohaven Pharmaceuticals
A study design with conditional, serially assessed coв•'primary endpoints: An application to a singleв•'arm, pilot non
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac

Impact of non‐binding FDA guidances on primary endpoint selection in Alzheimer's disease trials - Yu - 2022 - Alzheimer's & Dementia: Translational Research & Clinical Interventions - Wiley Online Library

Metastases-directed Radiotherapy in Addition to Standard Systemic Therapy in Patients with Oligometastatic Breast Cancer: Study protocol for a randomized controlled multi-national and multi-center clinical trial (OLIGOMA) - Clinical and Translational ...
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac
Sample size determination for a specific region in multiregional clinical trials with multiple co-primary endpoints
Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported rando
Design, data monitoring, and analysis of clinical trials with co-primary endpoints: A review: Journal of Biopharmaceutical Statistics: Vol 28, No 1